I. BACKGROUND
This paper presents and discusses continuous periodic muscle fasciculations observed in the avian trunk, a phenomenon not observed in mammals; and apparently attributable to the autonomic nerves, magnets and sensors monitoring multiple properties: potential, barometric pressure, acceleration, and optical reflex.
The global poultry industry (e.g., chicken, quail, ducks) involves some 20 billion individual animals, with an estimated market size of $ 120 billion per year. The type of the influenza (H1, H2, H3) responsible for global pandemics have been demonstrated to be transmitted directly to humans from birds via the low pathogenic avian virus. No genetic traces indicating transmission through swine have been detected in the past three pandemics.
The 2009 WHO declaration of a pandemic due to swine-derived viruses appears to have been significantly overestimated. The last novel influenza was of the swine type, and did not result in a pandemic, with mortality similar to that of seasonal influenza. The WHO Secretary General responsible for issuing the Pandemic Declaration was evacuated from the European Union. We need to pay close attention to the older concept s regarding flu policy [1]-[3].
Several reasons have been proposed to explain human infection from birds. Two explanations predominate:
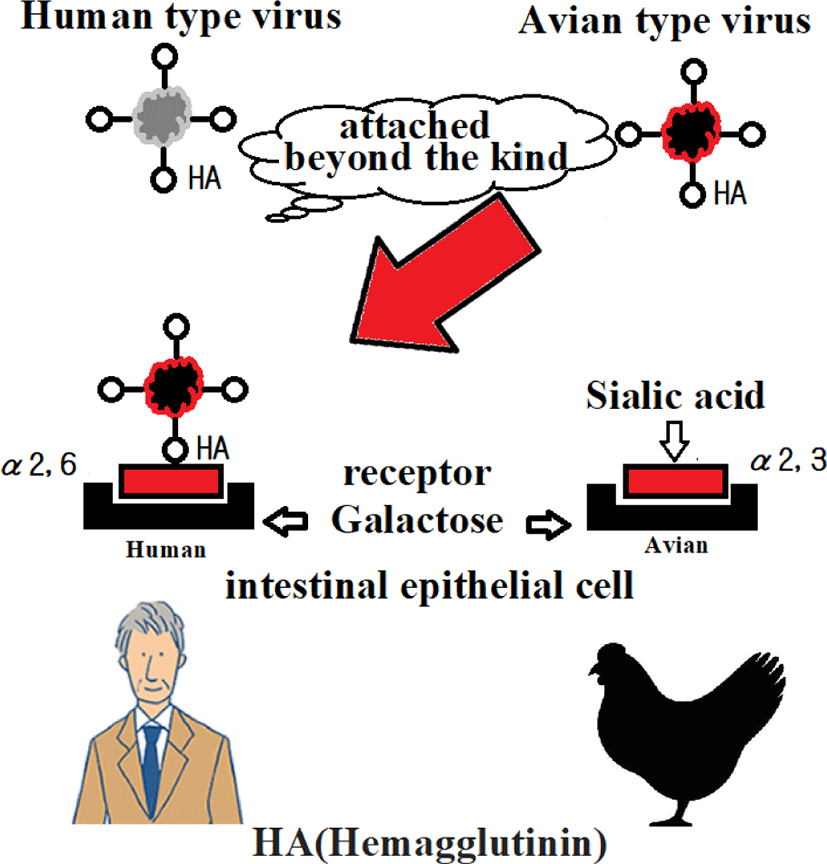
Human sialic acid receptor: Sialic acid is present in vivo at the end of sugar chains on the surface of cell membranes as a component of complex carbohydrates with long hydrocarbon chains called ceramides [4].
This acid has important biological functions. Of these, the sialic acid receptor targeted by the influenza virus in the early stages occurs most frequently in the respiratory system and digestive system. Since sialic acid is present at the tip of this complex carbohydrate, it is called a sialic acid receptor.
In fact, sialic acid receptors are found in abundance in α 2,3 or avian receptors in the deep lung (respiratory bronchioles and alveolar cells); α 2,6 is found in the upper respiratory tract. That is, there are mainly human receptors, especially in infants, and when infants are infected with H5N1, pneumonia becomes severe [5] (Figures 1 and 2).
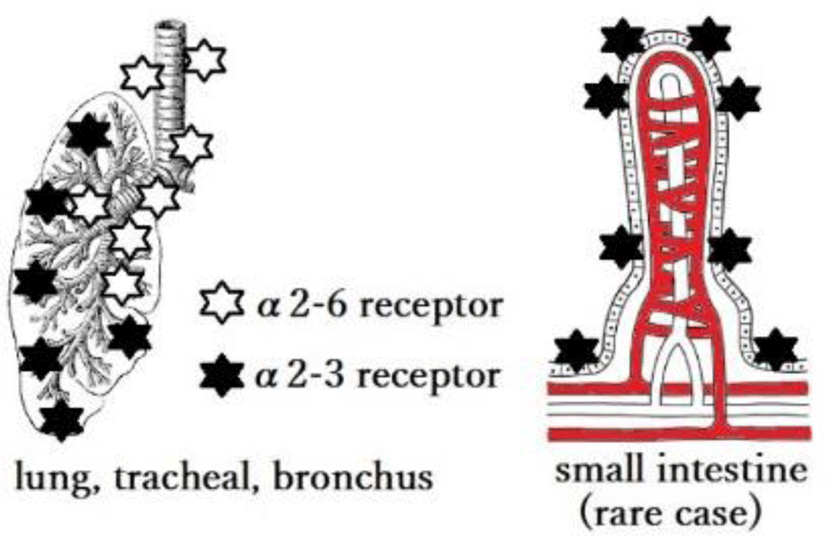
While, the frequency of appearance is relatively modest, α 2,3 type ebbs and flows in small intestinal epithelial cells over time. Human genes encode for both α 2,3 and α 2,6. This means that the earlier hypothesis that only pigs and ferrets(weasel) have both types and that viruses cannot infect humans through these intermediate hosts have not been substantiated. Recent studies indicate that H7N9 can hybridize with low-pathogenic viruses and seasonal influenza viruses to achieve human infectivity.
Birds do not have a diaphragm, and the position and size of the avian heart and the rotation of the aorta (not the left) differ markedly from those of mammals. Furthermore, as avian heart rates are extremely fast (200-400/min), it is necessary to devise measures such as time constants and measures against low potential when gathering data related to bird electrocardiograms. Infection by highly pathogenic avian influenza disturbs both breathing and he autonomic nerves (sympathetic and parasympathetic nerves), generating arrhythmias in electrocardiograms and ultimately, it is presumed, leading to cardiac arrest. Given this backdrop, we believe it is essential to screen affected individuals and to perform bird respiration monitoring and autonomic nervous system monitoring using ICT devices.
Against this background, we have developed an esophageal catheter to analyze the physiological functions of birds using multimedia technology, and wireless packet communication devices that attached wild whooper swans to collect their respiratory rate and heart rate [6-16]. In the past, physiological studies on birds and the relationship between autonomic nerves and electrocardiograms in chickens have been reported [17]-[27].
II. METHODS
Measurement uncertainty and noise can make it difficult to spot the oscillatory behavior of a signal, even if such behavior is expected. Several techniques are available for detecting periodic signals buried in noise. In this experiment, these five (electric potential, magnet, pressure, vibration, force) time-series-signals can be generalized as shown in the following equation:
Wt: Mean value =0, and follows a five-dimensional distribution of covariance matrix
This method calculates a statistic, making it possible to detect a signal of relatively strong amplitude. The drawback is that a signal with low amplitude will remain in the residual (Wt). We applied another approach, the classic frequency spectrum analysis method called FFT (Fast Fourier Transform). We selected a specific frequency band and reconstructed waveforms using IFFT (Inverse Fast Fourier Transform). Periodic signals detected in noise cannot be noise; additionally, if the signal is recognized in multiple dimensions and correlations (for example, in similar envelopes) are detected between the signals, an independent signal is likely to affect each dimension.
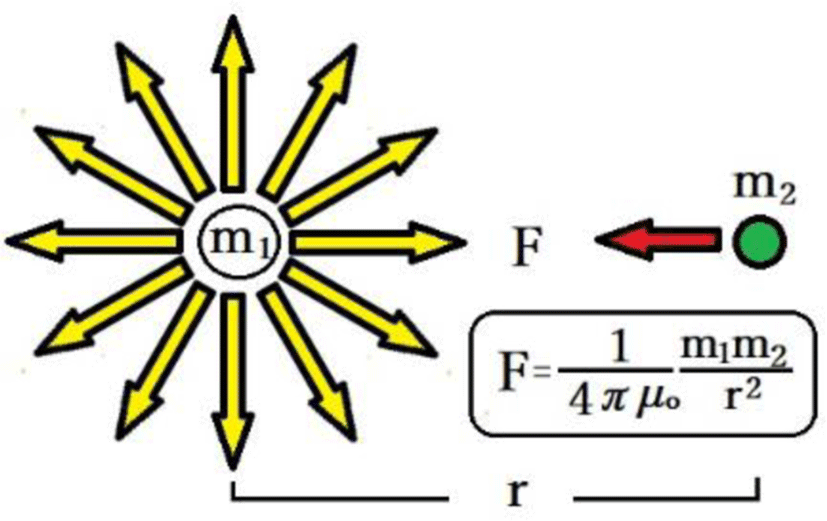
As discovered by Coulomb and summarized in Coulomb's law of electrostatic force, magnetic force acts between magnetic poles at magnitudes proportional to the product of the respective magnetic quantities m1 [Wb] and m2 [Wb] and inversely proportional to the square of the distance r [m] (Figure 3).
For p-type or n-type semiconductor samples, a current I is passed in the x direction and a magnetic field B is applied in the z direction (Figure 4). Charged particles (carriers) flowing through the sample have Lorentz force F due to the magnetic field and are accelerated along the y axis.
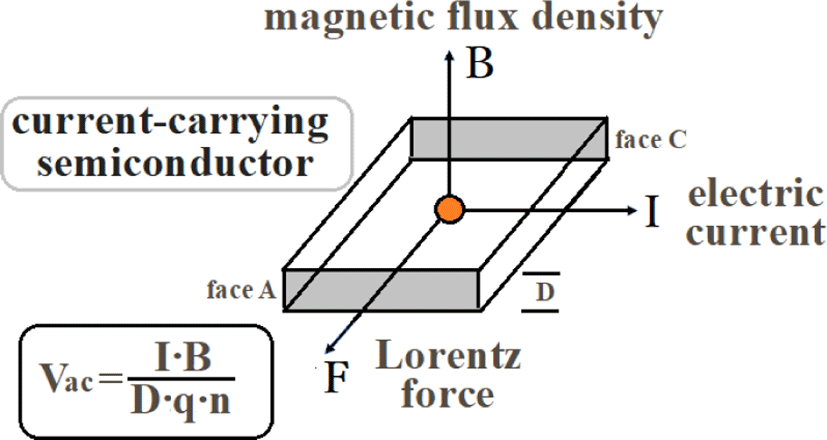
As a result, carriers accumulate on the surface of the sample, an electric field (Hall electric field) is generated in a direction orthogonal to both the current and the magnetic field, and an electromotive force Vac is generated. The Hall effect can be applied to the detection of the magnetic field strength by the Hall element.
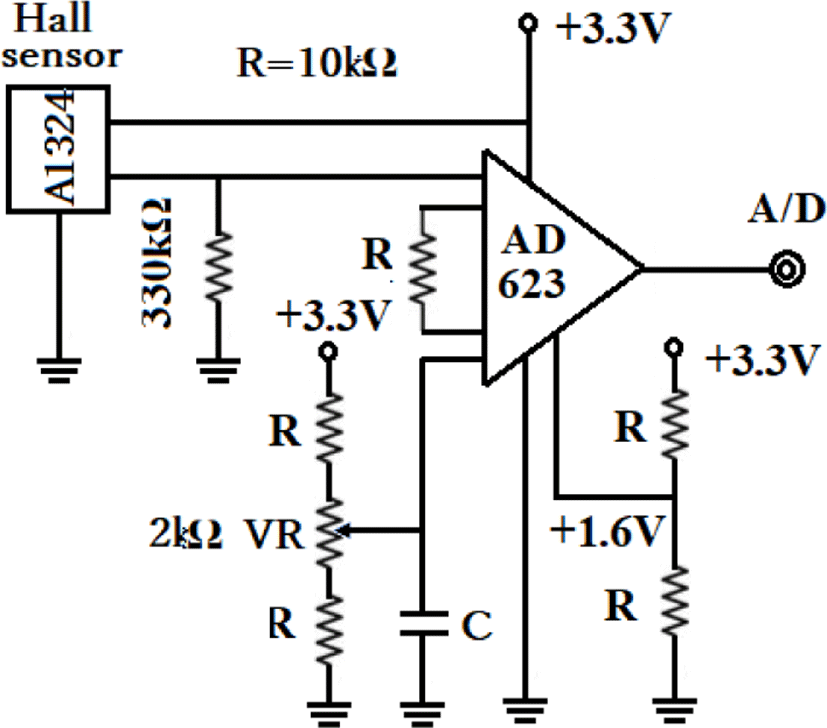
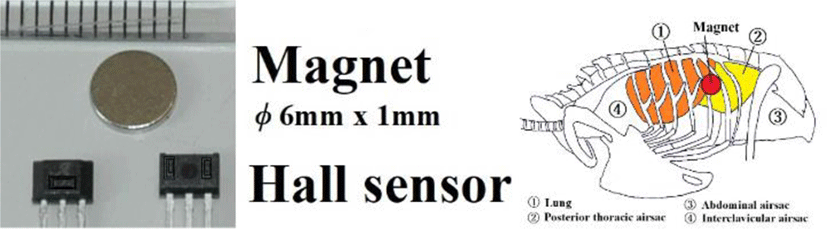
As mentioned above, force F is inversely proportional to the square of distance. If m1 and m2 are constant and the strength of the magnetic field changes, the distance has changed. In this experiment, changes in the magnetic field are measured using a semiconductor device called A132A, as the change in distance between two points is monitored. Figure 5 gives the circuit diagram.
If x (t) is a time signal and X (f) its frequency component, the Fourier series is expressed as the sum of the fundamental wave f0 and its integral frequency component. Applied here is a fast Fourier transform using butterfly computations, a division procedure. With FFT, time axis data for a certain time (frame) is cut out and calculated. The number of samples per frame must be a power of 2. Here, 1024 is one frame.
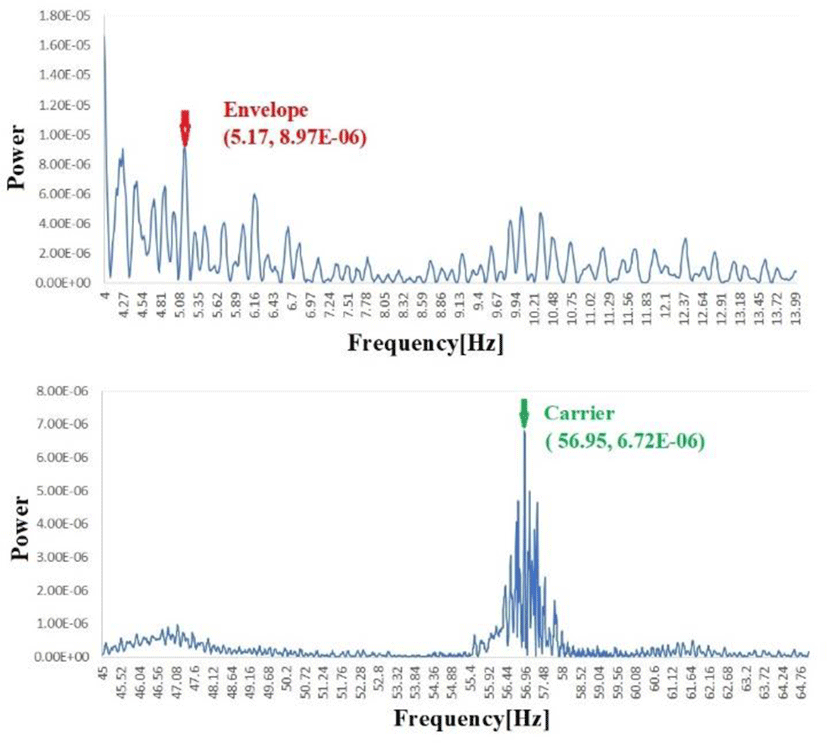
Sampling at 1,000 [Hz], a carrier frequency of 50-60 Hz amplitude, and an envelope (about 5-30 Hz) amplitude-modulated for that signal are used to resolve a specific frequency (e.g., 60 Hz for ECG reconstructed with IFFT or over 20 Hz for light reflections, depending on the site). This research seeks to confirm the source of this carrier signal. What does the source of this carrier signal and the meaning of the envelope?
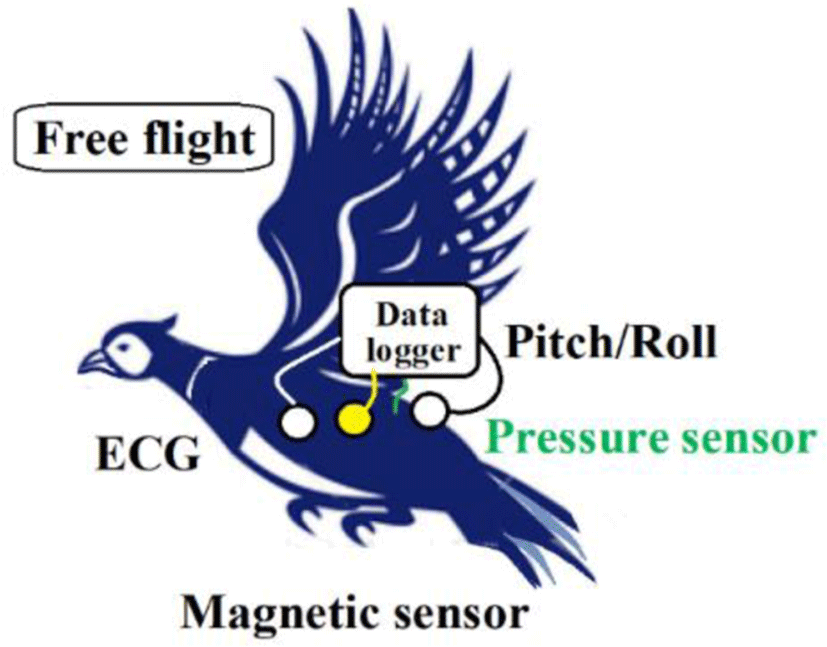
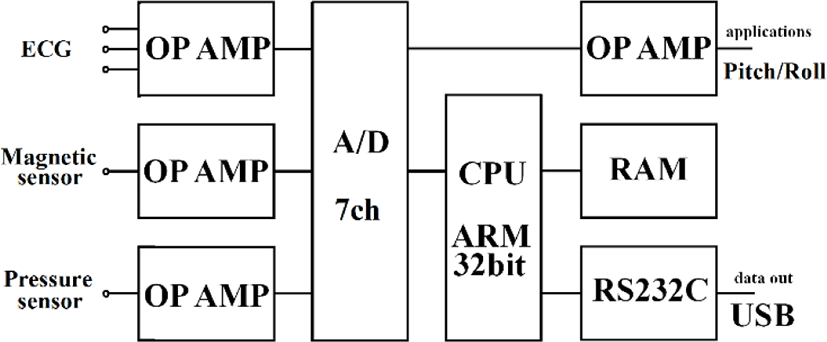
Physiological analyses of birds require signal processing technologies. We developed an ultra-compact data logger that birds are capable of carrying without impeding flight. Table 1 gives the specifications; Figure 6 shows a conceptual diagram; Figure 7 presents a block diagram.
| CPU | ARM 32 bit |
|---|---|
| Battery | 3.7 V |
| A/D conv. | A / D 14 bits, Max 1000 / sec. 7 ch.s |
| Memory | 100 MB |
| Data Output | Connected to the PC via USB |
| Total weight | 32 g |
The Hall sensor for magnetic field measurements and other devices were sewn to the pheasant as follows:
As shown in Figure 8, the magnet and Hall sensor were sewn with silk thread on the left and right thoracic walls (sixth intercostal space).
A bipolar electrocardiogram electrode (silver electrode, 8 mm diameter) was sewn subcutaneously on the head side (left and right chest walls) where the magnet was sewn. The neutral point was positioned under the midline of the pelvis.
A drain was inserted into the posterior chest air sac with a vinyl tube with an outer diameter of 2 mm. This was secured with silk thread, after which an atmospheric pressure sensor was connected. For intraoral pressure, a 50% area was partitioned in a 10 mm diameter cylinder. Air pressure was measured according to Bernoulli's theorem.
One angular velocity sensor (Murata ENC-03R) is based on the principle whereby the Coriolis force affects the rotational angular velocity applied to the vibrating body. This element was sewn with silk thread along the midline of the pelvis or midline of the back. The two are orthogonal and capable of detecting vibrations indicating pitch axis and roll axis.
Two LEDs (blue and green) covered with a black shield illuminate the side of the pelvis to create a structure that blocks direct light. Light reflected from the skin is received by an optical sensor and amplified. This is the same principle applied to detect SaO2. This optical reflection is considered to be transmitted through the skin and reflected from muscles.
III. EXPERIMENTS
-
- Female pheasants: 26; average weight 740 g
-
- Experiment place: Outdoor, tennis court (Oiso Campus, Seisa University)
Japanese pheasant females were chosen for the study because they are bred as poultry, readily available, and feature developed skeletal muscles, allowing relatively easy measurement of muscle signals. Figure 9 illustrates aspects of the flight experiment.
The results of the flight experiment are as follows. Periodic noise is observed for all pheasants at different frequencies at rest (in this case, before flight) and after flight.
Figure 10 shows the change in magnetic field strength detected by the Hall sensor. If we convert these signals by FFT and IFFT at high frequencies (higher frequency), they become third stage periodic noise. The lower area of Figure 10 shows an electrocardiogram recorded at the same site and at the same time. Both contain periodic noise that synchronizes, possibly due to the vibration of the intercostal muscles of the thoracic wall oscillating at a distance, which is riding as an amplitude modulation like AM radio signals. This phenomenon could be due to the intercostal muscle’s mechanical vibration(fasciculations).
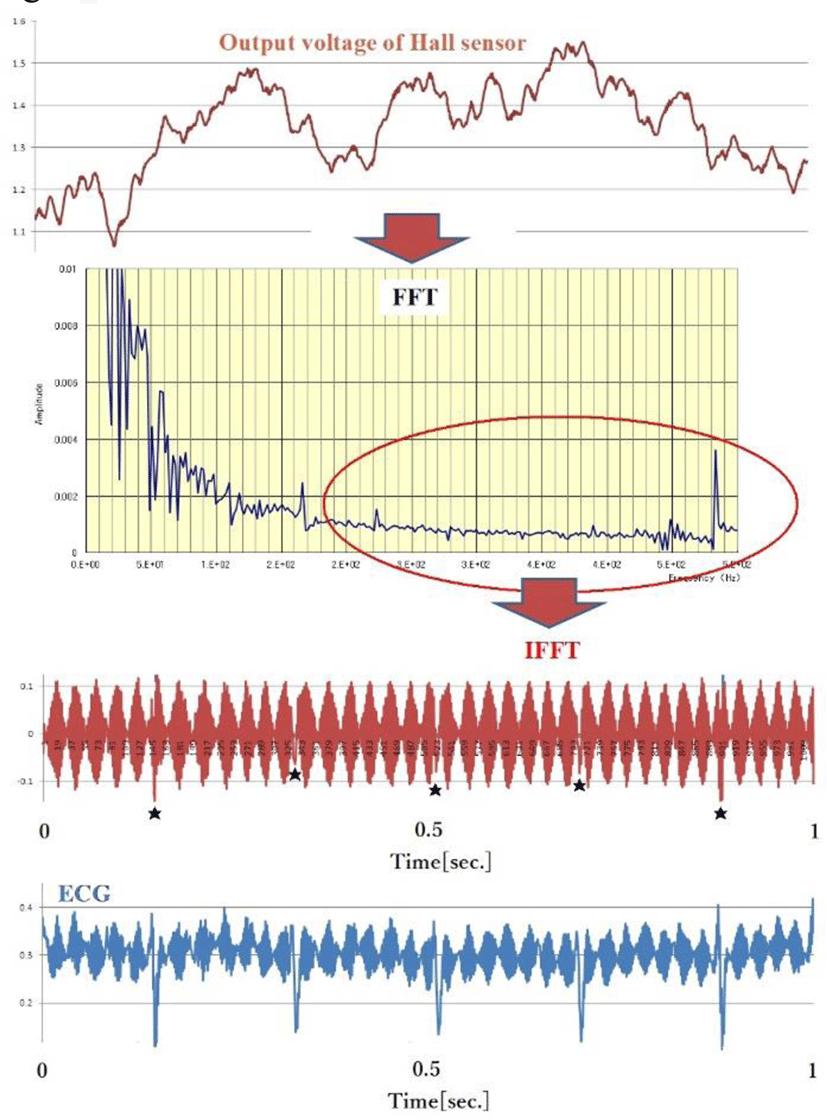
As shown in Figure 11, a periodic ECG electrode placed on the chest wall detects periodic noise, assumed to come from intercostal or other muscle’s electrical vibration (fasciculations).
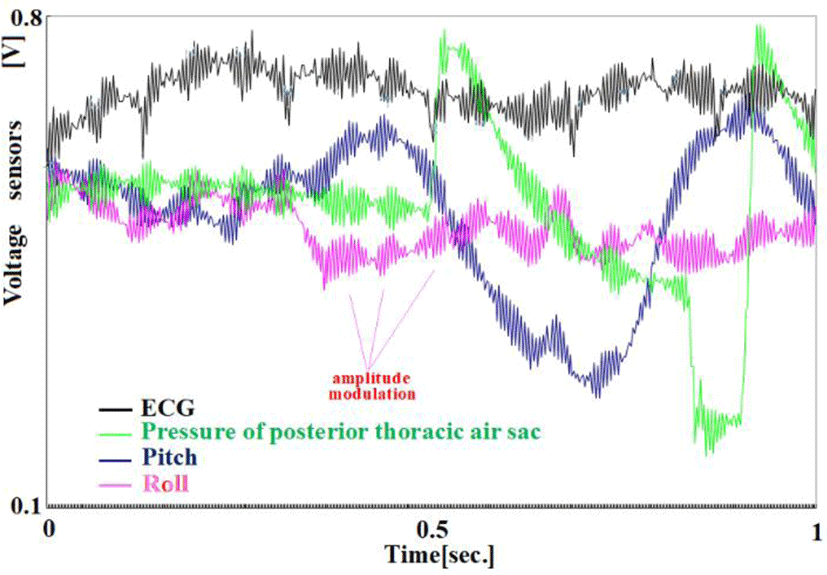
As shown in Figure 11, the pressure of the posterior thoracic air sac has a periodic signal that is amplitude-modulated by its pressure. This is believed to be due to the external tightening force generated by the upper pelvis muscle’s vibration (fasciculations).
As shown in Figure 11, vibrations affect both the pitch angle and the roll angle sewn on the chest wall and are synchronized with electrocardiogram noise. This vibration is believed to originate from intercostal muscles.
The light energy reflected by the skin (the side of the hindquarters) from the LED indicates periodic vibrations in both green and blue. The muscles on the side of the pelvis and the oblique muscles have a significant role in these vibrations(fasciculations).
Table 2 Frequency components and amplitude components before and after the flight compared with power spectrum analysis
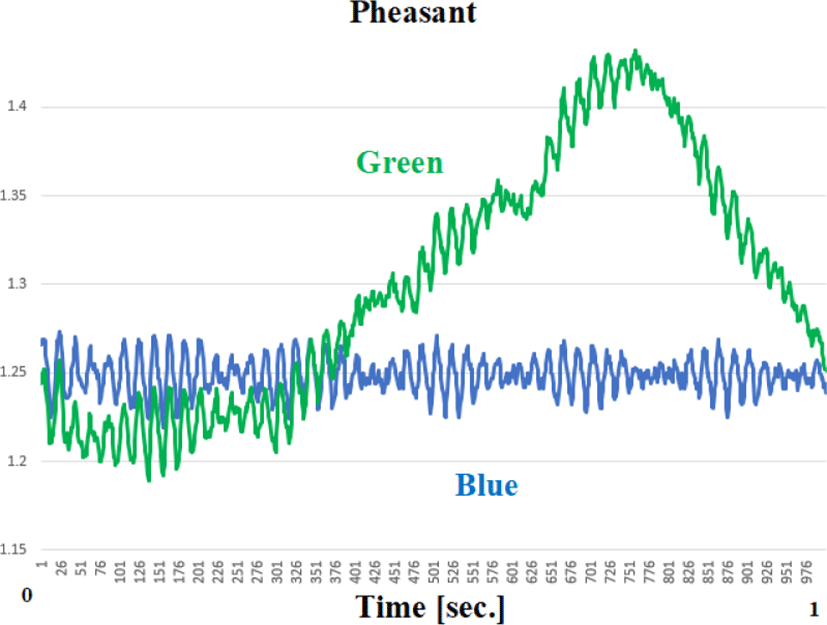
The frequency components and amplitude components of the envelope before and after the flight were compared with power spectrum analysis(Figure 11). Table 2 presents the amplitude of the peak of envelope before and after flight.
Based on these results, while the carrier frequency differs depending on the region (thoracic wall, pelvic, midline of back) and the action to be performed, periodic muscle contractions are evident in all cases.
These are buried deep in the noise and found in the frequency width removed by the low lass filter.
IV. CONSIDERATIONS
We derive the carrier frequency from the magnetic field (distance) between the thoracic walls, periodic noise found in the ECG, the angular velocity of the pelvic/thoracic wall, the periodic noise associated with air sac pressure, and optical waves reflected from the pelvic skin, all of which can be explained as originating from muscle vibrations. This low level of muscular tension is considered unique to birds, which lack diaphragms, and is deemed essential to maintaining constant air sac pressure. Current speculation is that such control systems are present in birds and dinosaurs, but not in mammals.
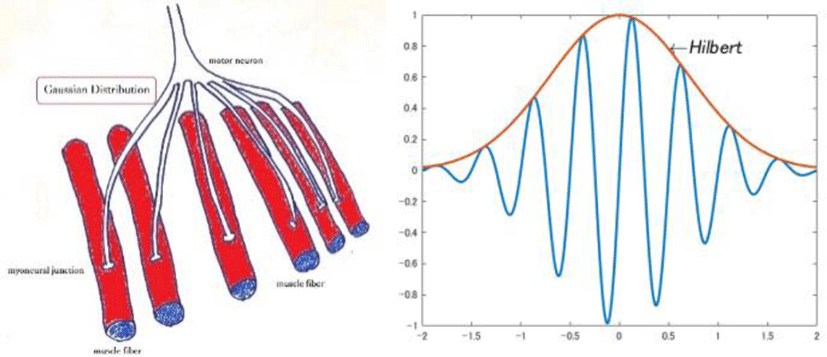
The number of envelopes is considered to represent a number of stimuli from the sympathetic nerve. Assuming that fibers from the sympathetic nerve are branched and spatially Gaussian (Figure 14) while the contraction of the muscle fibers is a trigonometric function, the observed signal is a convolution signal of both. The shape is similar to the noise and optical reflection contained in the ECG. This convolution can be simulated with a relatively simple program (Annex 1) in MALBAT.
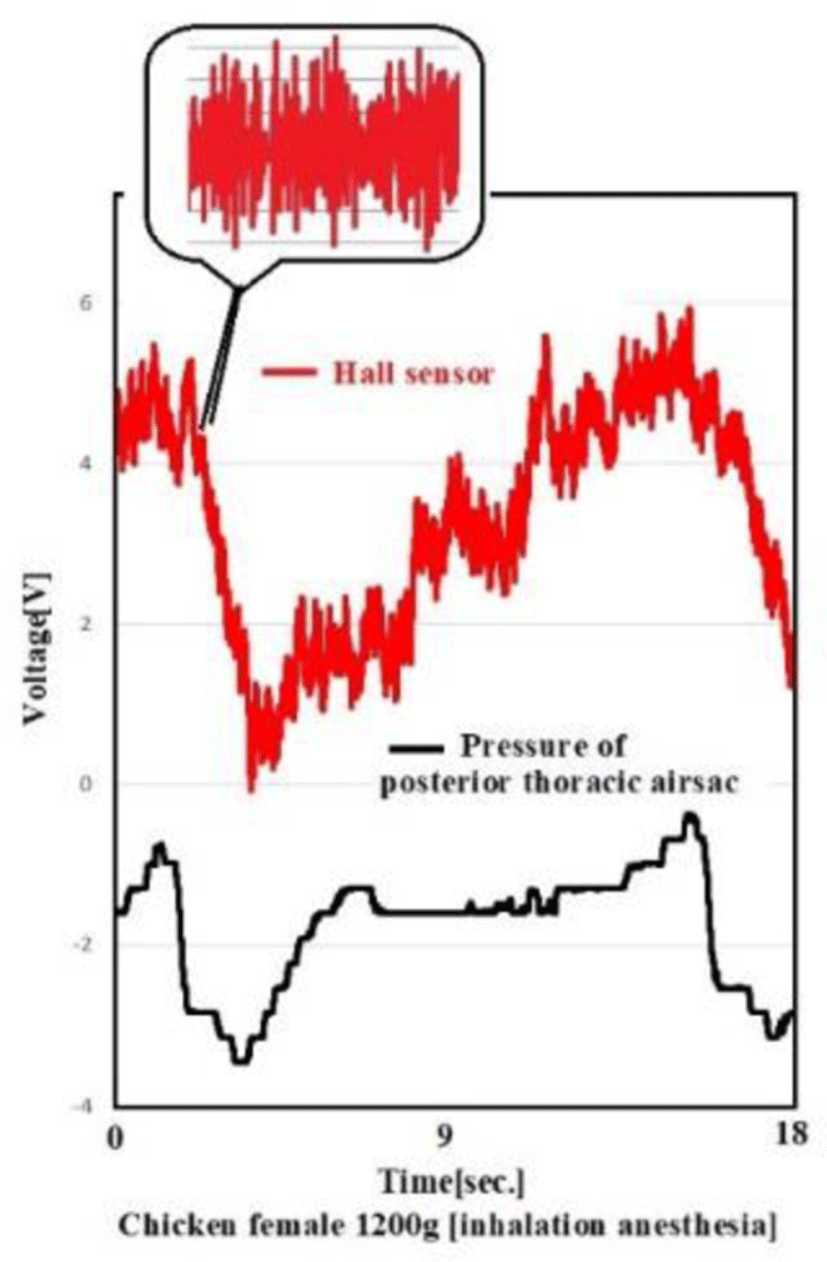
In an experiment using chickens, a breathing pattern was obtained using magnets attached to the thoracic wall, which were synchronized with posterior thoracic air sac pressure (with a certain time difference). Also observed were periodic oscillations of sympathetic nerve-derived intercostal muscles. This approach allows 24/365 monitoring of a chicken's respiratory status and autonomic state without contact.
As shown in Figure 15, the posterior thoracic air sac pressure and changes in the magnetic field of the thoracic wall are correlated. The respiratory patterns of the chicken and periodic fasciculations of intercostal muscles are clearly recorded.
Poultry tend to be potential low-pathogenic avian influenza carriers with the potential of mutation to high pathogenicity. Nevertheless, visually identifying birds in poor health remains difficult. We believe detecting antigen-antibody reactions from a dead bird, based on refinements of this technology, will enable proactive crisis management that screens for suspicious individuals at an early stage and isolates them from the population, an approach preferable to culling after the fact.
Especially during forced molting (forcing starvation and measures to promote ovulation in groups that no longer lay eggs), vital signs fluctuate greatly, with many individual birds developing Newcastle disease. This study is based on the pathophysiology of avian influenza. In place of antigen-antibody reactions, respiratory patterns are detected without contact using a magnet attached to the thoracic wall and a Hall sensor attached to the cage wall. This allows isolation of individual birds in the early stages of infection. Linked to a data line, this will allow local governments to monitor conditions and provide suitable guidance (Figure 16). We see this early isolation as one solution for preventing the spread of avian influenza.
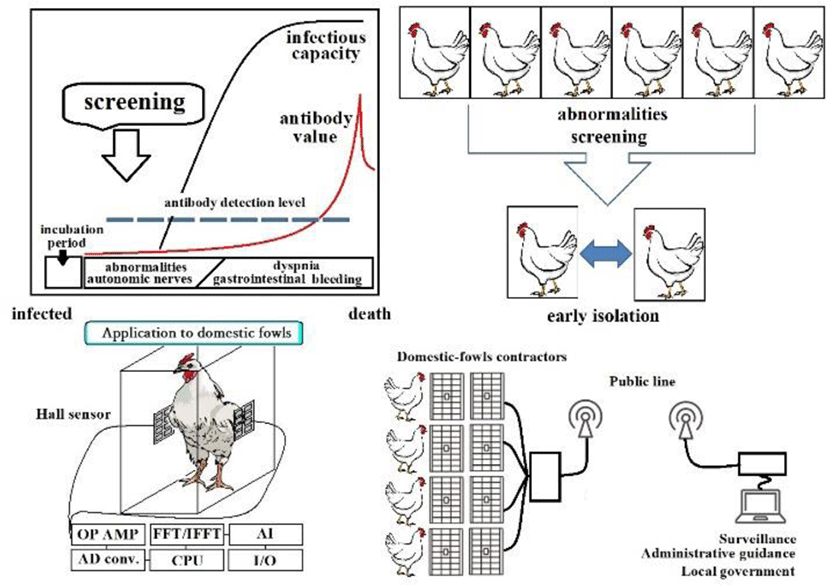
There are two methods of detecting abnormalities with magnets: (a) respiratory patterns and (b) periodic fasciculations.
(a)Can the presence or absence of a notch be detected by respiratory pattern? This notch is caused especially airflow between the air sacs. This detection can be enhanced with AI (Recurrent Neural Networks). For example, with RNN, learn the normal state 1000 times, and then learn 1000 times with the low oxygen inhalation condition intentionally, in the next tern 2001, the pattern is healthy or disorder?
(b)For periodic fasciculations, which reflect the condition of sympathetic nerves, the frequency of the envelope and the strength of the carrier frequency. When the disease develops, the frequency of the envelope and the signal strength decreases.
Up to now, there is no system to monitor the vital signs of birds from the poultry house in real time. Poultry traders are owners and do not report dead bodies and sometimes sell them. This is why governments need a system to manage poultry remotely.
IV. CONCLUSION
The following is a summary of this paper:
-
Magnets and Hall sensors sewn on the left and right thoracic walls, bipolar electrocardiograms measured between same thoracic walls, posterior thoracic air sac pressure, angular velocity sensors sewn on the back or hips, and optical reflection of LEDs (blue and green) from the skin of the hips make it possible to observe periodic vibrations (fasciculations). To date, no such analysis has been reported.
-
These fasciculations are presumed to derive from muscle, with the function of maintaining and controlling air sac pressure.
-
Since each muscle fiber is a spatial Gaussian distributed from the sympathetic nerve, it is assumed that the envelope plots a Gaussian curve.
-
Avian trunk muscles contract periodically at all times, suggesting that the sympathetic nerve controlling this is predominant.
-
The technique of sewing a magnet to the thoracic wall and measuring the strength of magnetic fields with a Hall sensor can be applied to captive animals to allow screening for the early stages of avian influenza.
the Gaussian convolution and its Envelope
% Open source code of MATLAB for readers of J of Multimedia Information System
% Isao Nakajima, Tokai University, Nov. 30, 2019
Fs = 100; % sampling frequency
x = -2:1/Fs:2; % vector of time
y = cos(4*pi*x-(pi/2)).*exp(-x.^2); % Gaussian distribution
z = hilbert(y); % Hilbert
w = abs(z);
plot(x,y,x,w,'LineWidth',2)
text(0.495, 0.8,'\leftarrow\it{Hilbert}','FontSize', 16)
filename='E:\Hilbert.csv';% save the envelope
str = [x;w;]
csvwrite(filename,str)
type('C:\Hilbert.csv')